Abstract
Background:
AL amyloidosis, a rare, severe, progressive, systemic disorder caused by plasma cell dyscrasia (PCD), results in insoluble immunoglobulin light chain amyloid fibrils depositing in organs and causing significant dysfunction, morbidity, and mortality.
Most patients receive anti-PCD therapy as standard of care (SOC) to suppress plasma cell proliferation and arrest the generation and deposition of new amyloid fibrils. At present, no approved therapies exist that target fibrils already deposited.
CAEL-101, a monoclonal antibody, binds to amyloid light chain fibrils and promotes removal from tissues. In this Phase 2 trial, patients were treated with doses up to 1000 mg/m 2, combined with SOC, demonstrating this dose was well tolerated and appropriate for Phase 3.
Aim:
Evaluate long-term safety and tolerability of CAEL-101, administered with SOC in AL amyloidosis.
Methods:
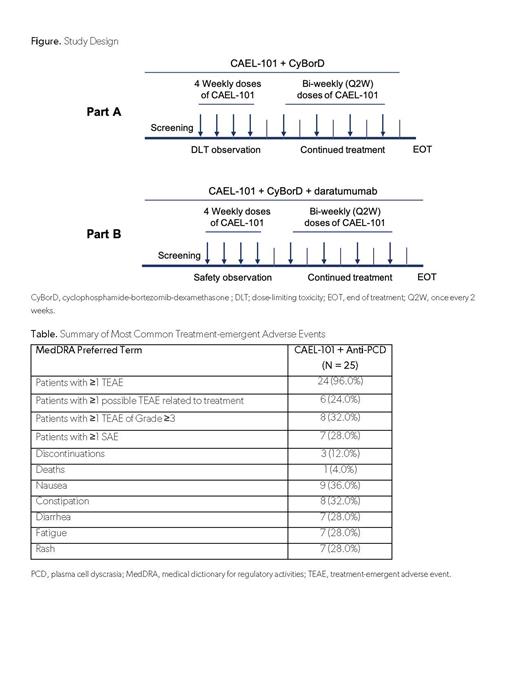
Adult patients with confirmed AL amyloidosis diagnosis (Mayo Stages I, II, IIIa), 6-month minimum life expectancy, and measurable hematologic disease were eligible for this ongoing, open-label, phase 2 study (NCT04304144). Patients with other forms of amyloidosis, multiple myeloma, supine systolic blood pressure <90 mm Hg, or symptomatic orthostatic hypotension were excluded. All patients received CAEL-101 1000mg/m 2 every other week with SOC anti-PCD therapy until investigator decided anti-PCD was no longer needed (Figure).
Safety assessments included treatment-emergent adverse events (TEAEs), clinical laboratory tests, electrocardiograms, vital signs, and physical examinations. Pharmacokinetic endpoints included maximum serum concentration (C max) and minimum serum concentration of CAEL-101 prior to next dose (C trough). Exploratory endpoints included biomarkers for cardiac function (cardiac troponin T [cTnT] and N-terminal pro-brain natriuretic peptide [NT-proBNP]), and renal function (estimated glomerular filtration rate and proteinuria).
Results:
The 25 patients averaged 65.2 years (range 47 to 80), with the majority male (72.0%). Mayo Stages I (8.0%), II (76.0%), and IIIa (16.0%) reflected the wide range of disease severity in enrolled patients ; 19 (76.0%) presented with cardiac involvement, 8 (32.0%) with renal involvement, and 20 (80.0%) had received prior anti-PCD therapy.
Twenty-four (96.0%) patients experienced TEAEs, but only 6 (24.0%) experienced a possibly treatment related TEAE (Table). Eight (32.0%) patients experienced at least 1 Grade ≥3 TEAE and 7 (28.0%) experienced at least 1 serious adverse event. There were 3 (12.0%) discontinuations; 1 death due to septic pneumonia (investigator determined not related to CAEL-101), one heart transplant, and one patient who withdrew consent. Most common TEAEs included nausea (9 [36.0%]], constipation (8 [32.0%]), and diarrhea, fatigue, or rash (7 [28.0%] each).
Addition of daratumumab (n = 12) to the anti-PCD combination treatment of cyclophosphamide-bortezomib-dexamethasone (CyBorD) did not alter the pharmacokinetic or tolerability profile of CAEL-101.
Of the 19 current cardiac evaluable patients (baseline NT-proBNP ≥332 ng/L and ≥1 post-first-dose NT-proBNP value), 15 (78.9%) have responded (≥ 30% NT-proBNP decrease from baseline) or are stable on CAEL-101 therapy. Renal evaluable patients, as determined by Investigator at a single site, showed a similar proteinuria response.
Discussion:
This ongoing trial is evaluating the long-term safety and tolerability of CAEL-101 administered with anti-PCD SOC as a treatment to reduce amyloid burden in patients with cardiac AL amyloidosis. CAEL-101 was well tolerated when administered with anti-PCD therapy. Most TEAEs observed were mild to moderate in severity and did not require intervention. There were no meaningful differences in tolerability or exposure to CAEL-101 when daratumumab was added to the anti-PCD regimen. Improvements in cardiac and renal response biomarkers were observed in most patients presenting with cardiac or renal involvement, respectively, at study entry.
Conclusion:
After approximately 1-year, CAEL-101, as part of an AL amyloidosis treatment strategy, demonstrates to be well tolerated. This updated report confirms previous findings for the use of CAEL-101 in combination with anti-PCD. A Phase 3 clinical program is ongoing to further elucidate the efficacy and safety of CAEL-101.
Valent: Takeda Pharmaceuticals: Speakers Bureau; Amgen: Speakers Bureau; Caelum Biosciences: Other: Clinical Trial Funding; Celgene Corporation: Speakers Bureau. Zonder: Caelum Biosciences: Consultancy; Regeneron: Consultancy; Intellia: Consultancy; Amgen: Consultancy; Janssen: Consultancy; Takeda: Consultancy, Membership on an entity's Board of Directors or advisory committees; Alnylam: Consultancy; BMS: Consultancy, Research Funding. Liedtke: Sanofi: Membership on an entity's Board of Directors or advisory committees; Takeda: Membership on an entity's Board of Directors or advisory committees; Kura Oncology: Membership on an entity's Board of Directors or advisory committees; Pfizer: Honoraria; Oncopeptides: Membership on an entity's Board of Directors or advisory committees; Celgene: Membership on an entity's Board of Directors or advisory committees; GlaxoSmithKline: Membership on an entity's Board of Directors or advisory committees; Janssen Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees; Karyopharm: Membership on an entity's Board of Directors or advisory committees; Kite: Membership on an entity's Board of Directors or advisory committees; Caelum: Membership on an entity's Board of Directors or advisory committees, Other: Clinical Trial Funding; Bristol Myers Squibb: Membership on an entity's Board of Directors or advisory committees; Alnylam: Membership on an entity's Board of Directors or advisory committees. Silowsky: Caelum Biosciences: Current Employment. Kurman: Caelum Biosciences: Other: Medical Monitor. Daniel: Caelum Biosciences: Current Employment. Jobes: Caelum Biosciences: Current Employment. Harnett: Caelum Biosciences: Current Employment. Raviwong: Caelum Biosciences: Current Employment. Spector: Caelum Biosciences: Current Employment. Sobolov: Caelum Biosciences: Current Employment.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal